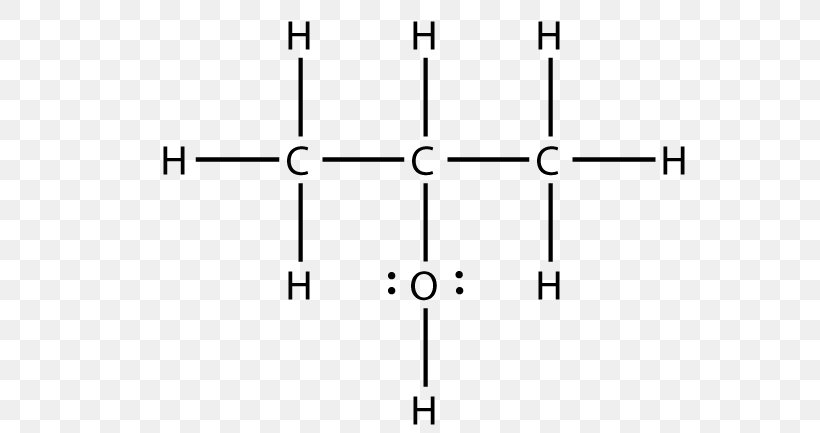
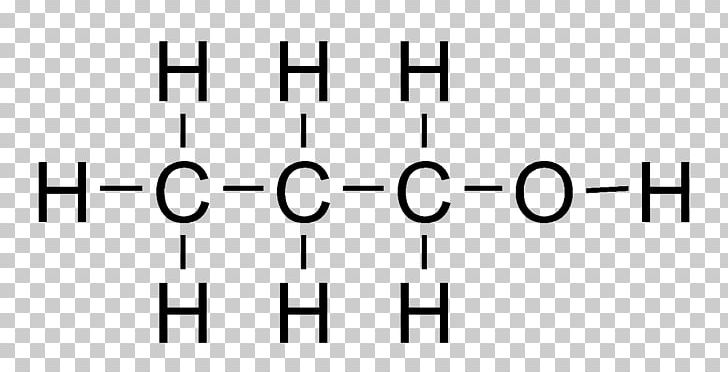
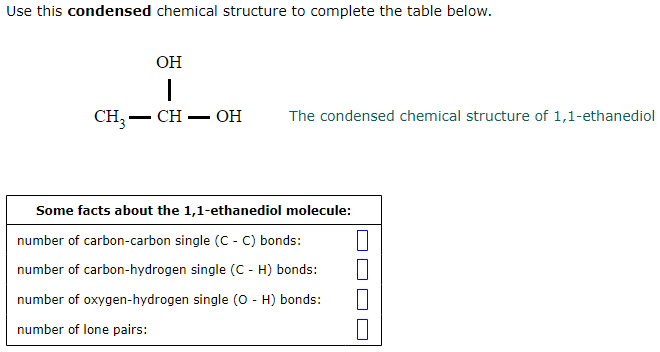
Page 3 of 13 Action of the Substance 90 91 Ethylene glycol dissolves in water and is miscible in alcohol and acetone, has the capability to hold large 92 amounts of heat before boiling, and lowers the freezing point of water (Lewis,Figure 1 Uses of ethane1,2diol Data for 13 from a variety of sources including IHS Markit, 16 By far the most important uses of the diol is in the manufacture of polyesters, particularly PET (polyehylene terephthalate), used widely for clothes and for packaging Indeed 45% of the polyester is used for bottles 1 1 Global Processing, 16The condensed chemical structure of 1,1ethanediol Some facts about the 1,1ethanediol molecule number of carboncarbon single bonds number of carbonhydrogen single bonds number of oxygenhydrogen single bonds number of lone pairs Chemists often draw the Lewis structure of organic compounds like 1,1ethanediol in a "condensed " style normal condensed In the

Ch3ch2oh Lewis Structure How To Draw The Lewis Structure For Ch3ch2oh Youtube
1 1-ethanediol lewis structure
1 1-ethanediol lewis structure- What is the structural formula for 1/2 Ethanediol?Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxideEthylene oxide reacts with water to produce ethylene glycol according to the chemical equation C 2 H 4 O H 2 O → HO−CH 2 CH 2 −OH This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures




1 1 Ethanediol Structure C2h6o2 Over 100 Million Chemical Compounds Mol Instincts
Examples include 1,2ethanediol (ethylene glycol, used in antifreeze) and 1,2,3propanetriol (glycerine, used as a solvent for cosmetics and medicines) Write two complete balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structuresEw of a molecule of (4 S ,5 S )2,2dimethyl1,3dioxolane4,5dicarbonitrile indicating the atom numbering scheme Thermal ellipsoids are drawn at the 50% probability levelHow is vsepr used to classify molecules?
Chemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Species with the same structure Pluronic f68We report here on new ligands based on a set of simple diols that had been previously overlooked Ligands based on (S,S)‐trans‐cyclohexanediol and (R,R)‐()‐1,2‐diphenyl‐1,2‐ethanediol, in combination with both chiral and achiral amines, were tested in 3 different copper‐catalyzed asymmetric reactions and up to % ee was observed Examplestrans1,2dichloro1,2ethanediol (meso)2,3dibromobutane Answer link Related questions How do I determine the molecular shape of a molecule?
I quickly take you through how to draw the Lewis Structure of CH3CH2OH (Ethanol) I also go over hybridization, shape, sigma, pi bonding and bond anglesXeO 2 F 2 Steps for Writing Lewis Structures Find the total valence electrons for the molecule Explain How Examples H 2 S, NCl 3, OH Put the least electronegative atom in the center Note H always goes outside Examples NOCl, CF 2 Cl 2, HCN1,3Dioxanes, 1,3Dioxolanes WileyInterscience, New York, 1999, 3022, 1,3Dioxanes and 1,3dioxolanes can easily be prepared from carbonyl compounds with 1,3propanediol or 1,2ethanediol in the presence of a Brönsted or a Lewis acid catalyst 1,3Diols give more stable compounds A standard procedure for protection employs



Ethane 1 2 Diol Formaldehyde C3h8o3 Pubchem




What Is The Electron Dot Structure Of Ethane Quora
(Hint How are the hydrogen atoms on different carbons oriented with respect to each oth Change ethane into 1,2ethanediol (HOCH2CH OH) by removing one hydrogen atom from each carbon atom and replacing with a hydroxide (OH) group 3 Draw a Lewis structure for 1,2ethanediol 4 Build a model of the 1,2ethanediol molecule aA stepbystep explanation of how to draw the C3H8 Lewis Dot Structure (Propane)For the C3H8 structure use the periodic table to find the total number of vaAcademiaedu is a platform for academics to share research papers



2



1
To embed a widget in your blog's sidebar, install the WolframAlpha Widget Sidebar Plugin, and copy and paste the Widget ID below into the "id" field To add a widget to a MediaWiki site, the wiki must have the Widgets Extension installed, as well as the code for the WolframAlpha widget To include the widget in a wiki page, paste the codeWhat is the lewis structure for hcn?Its molecular formula is CCl3CH(OH)2 and its molecular weight is 1654 It occurs as colorless or white, volatile, hygroscopic crystals very soluble in water and in olive oil and freely soluble in alcohol It has an aromatic, pungent odor and a slightly bitter, caustic taste



چین Cas 60 10 6 تولید کنندگان Dithizone نمونه رایگان Alfa Chemical



2
B 1, 2ethanediol c 5methyltrans2heptene Structures of Organic Compounds The structures of organic compounds can be represented as either Lewis structures, Kekule structures, condensedThe –OH group in an alcohol molecule is attached to a carbon atom by a covalent bond Ethanol, CH 3 CH 2 OH, also called ethyl alcohol, is a particularly important alcohol for human use Ethanol is the alcohol produced by some species of yeast that is found in wine, beer, and distilled drinksEthanethiol, commonly known as ethyl mercaptan and stench, is a clear liquid with a distinct odor It is an organosulfur compound with the formula CH3CH2SH Abbreviated EtSH, it consists of an ethyl group, CH3CH2, attached to a thiol group, SH Its structure parallels that of ethanol, but with sulfur in place of oxygen The odor of EtSH is infamous Ethanethiol is more volatile than ethanol due to




Zeolite Supported Rhenium Catalysts For The Deoxydehydration Of 1 2 Hexanediol To 1 Hexene Meiners 21 Chemcatchem Wiley Online Library




Ch3ch2oh Lewis Structure How To Draw The Lewis Structure For Ch3ch2oh Youtube
The polyol process is a generic method for metal nanoparticle synthesis It consists of a reduction of metal salts in a liquid αdiol such as 1,2ethanediol (ethylene glycol), 1,2propane diol (propylene glycol) or 1,2butanediol These liquids exhibit a high polarity that allows the dissolution of metal salts, a high boiling point and anStructure, properties, spectra, suppliers and links for 1,2EthenediolCAS Registry Number ;



Methanol




Alcohols And Ethers
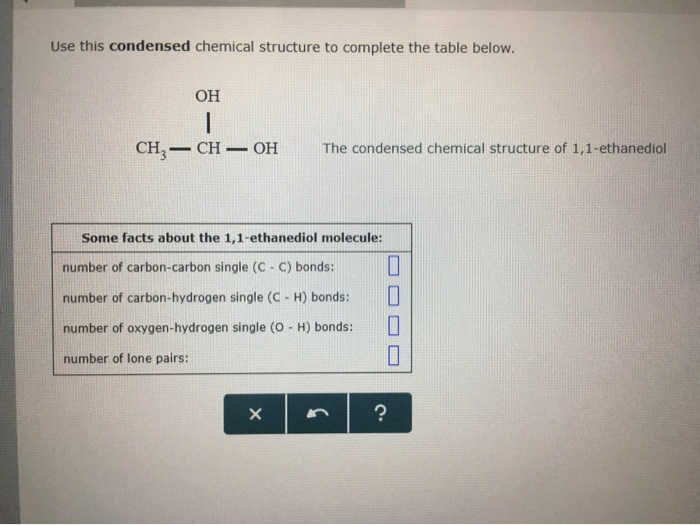
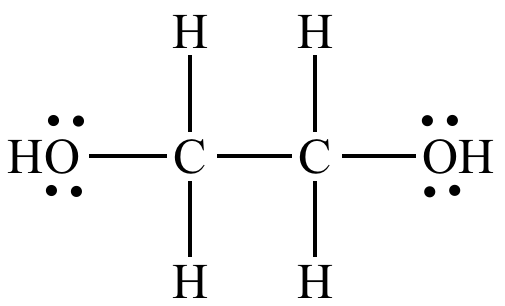
Use this condensed chemical structure to complete the table below OH CH2 – CH – OH The condensed chemical structure of 1,1ethanediol Some facts about the 1,1ethanediol molecule number of carboncarbon single (CC) bonds number of carbonhydrogen single (CH) bonds number of oxygenhydrogen single (0 1) bonds number of lone pairs xs ?Structure, properties, spectra, suppliers and links for 1,1Ethanediol,C2H6O2 How many lone pairs are in ch3ch2oh?



Chloral Hydrate C2h3cl3o2 Chemspider




Pushing Curly Arrows
The rate constant for the vaporphase reaction of ethylene glycol with photochemicallyproduced hydroxyl radicals has been reported as 77X1012 cu cm/moleculesec at 25 °C (1) This corresponds to an atmospheric halflife of about 50 hours at an atmospheric concentration of 5X105 hydroxyl radicals per cu cm (2)The ChemDoodle Web Components library is a pure JavaScript chemical graphics and cheminformatics library derived from the ChemDoodle application and produced by iChemLabs ChemDoodle Web Components allow the wielder to present publication quality 2D and 3D graphics and animations for chemical structures, reactions and spectra Beyond graphics, this tool Vaporphase dehydration of several 1,2alkanediols, such as 1,2ethanediol, 1,2propanediol, 1,2butanediol and 1,2pentanediol, to produce corresponding aldehydes was investigated over silicasupported WO 3 catalyst, which was prepared by impregnation method and then calcined at 3 °C Higher than 90% yield of aldehydes could be achieved over WO 3 /SiO 2



Staggered Vs Eclipsed Conformations Of Ethane Master Organic Chem



Rcsb Pdb 4o5j Crystal Structure Of Saba From Helicobacter Pylori
Answer (1 of 3) Water is made of 2 hydrogen atoms bonded to a single oxygen atom The oxygen atom has two available bond locations, one for each hydrogen This makes one water molecule, HOH (or H2O) Drinking alcohol (aka ethyl alcohol or ethanol) isSyst IUPAC Name 1,2ethanediol Syst IUPAC Name _____ (b) Ethanol is soluble with water in any ratio In an aggregate formed by one ethanol molecule and one water molecule, ethanol can act as hydrogen bond acceptor or as a hydrogen bond donor Draw the complete Lewis structures (all atoms, all lone pairs) of both aggregatesAlcohols containing two or more hydroxyl groups can be made Examples include 1,2ethanediol (ethylene glycol, used in antifreeze) and 1,2,3propanetriol (glycerine, used as a solvent for cosmetics and medicines) Naming Alcohols The name of an alcohol comes from the hydrocarbon from which it was derived




Competing Intramolecular Vs Intermolecular Hydrogen Bonds In Solution Abstract Europe Pmc




Influence Of Interparticle Electronic Coupling On The Temperature And Size Dependent Optical Properties Of Lead Sulfide Quantum Dot Thin Films Journal Of Applied Physics Vol 119 No 9
What is the lewis structure for co2?Alcohols, represented by the general formula C n H 2n1 OH, are organic compounds with a hydroxy group attached to an sp 3hybridized carbon of an aliphatic system They exhibit a bent geometry around the oxygen atom, where the sp 3 hybrid orbital of oxygen overlaps with the sp 3 hybrid orbital of carbon, forming a sigma bond The carbon–oxygen–hydrogen bond angleEthanol's hydroxyl group causes the molecule to be slightly basic



Solved Use This Condensed Chemical Structure To Complete The Chegg Com



1
This information is only displayed if the substance is welldefined, its identity is not claimed confidential and there is sufficient information available in ECHA's databases for ECHA's algorithms to generate a molecular structure More help available here EC / List no CAS no Mol formula C2H6O2Reactions with 1,2ethanediol, 1,2propanediol, glycolic acid, lactic acid, and oxalic acid are reported The successive reactions of the diols with B(OH)4 are shown toStep 1 Protonation of phenylethanone (acetophenone) by sulphuric acid to give an oxonium ion Step 2 A resonance structure of this species which is a carbocation Step 3 Attack of water (as a nucleophile) on this carbocation to give a new oxonium ion Step 4 Abstraction of a proton by water (as a base) to produce 1phenyl1,1ethanediol Draw the other important resonance



Ethylene Glycol Chemical Formula And Structure



E Organic Chemistry Exercises Chemistry Libretexts
Chemically, chloral hydrate is 1,1Ethanediol, 2,2,2trichloro;Ethane1,2diol CAS Number Chemical Formula C 2 H 6 O 2 click here for details WEB SEARCH MSDS RESOURCES SUPPLIERSExamples include 1,2ethanediol (ethylene glycol, used in antifreeze) and 1,2,3propanetriol (glycerine, used as a solvent for cosmetics and medicines) one using condensed formulas and one using Lewis structures (a) 2butene is treated with water in dilute acid (b) ethanol is dehydrated to yield ethene Glossary




Interpreting Condensed Chemical Structures Aleksmeaghane Troy4 25 Meaghane Troy4 25 pmest Course Hero




Rcsb Pdb 6sp6 Ultra High Resolution Crystal Structure Of The Ctx M 15 Extended Spectrum Beta Lactamase In Complex With Taniborbactam Vnrx 5133
CAS No Ethylene glycol (HOCH ₂ CH ₂ OH) is a colorless, syrupy liquid It can harm the eyes, skin, kidneys, and respiratory system Ethylene glycol can cause death if swallowed Workers may be harmed from exposure to ethylene glycol The level of exposure depends upon the dose, duration, and work being doneChange ethane into 1,2ethanediol (HOCH 2 CH 2 OH) by removing one hydrogen atom from each carbon atom and replacing with a hydroxide (OH) group 3 Draw a Lewis structure for 1,2ethanediolAccording to Lewisdot structure, there are 16 number of bonding electrons and 4 number of nonbonding electrons or lone pair of electrons Why does ethanol have a pH of 7?




Prediction Of Partial Miscibility Of Binary Mixtures Consisting Of Ionic Liquids Journal Of Thermophysics And Heat Transfer
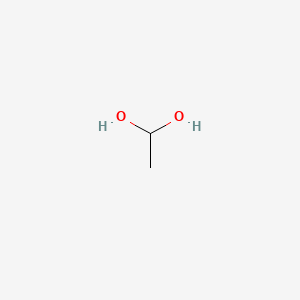



1 1 Ethanediol C2h6o2 Pubchem
Poly (di1H, 1H, 2H, 2Hperfluoroalkylitaconate) films surface organisation phenomena, surface energy determinations and force of adhesion measurementsEthylene glycol, C 2 H 6 O 2, has one OH bonded to each carbon (a) Draw the Lewis dot structure of ethylene glycol (b) Draw the Lewis dot structure of chloroethane, C 2 H 5 Cl (c) Chloroethane has a slightly higher molar mass than ethylene glycol, but a much lower boiling point (3 °C versus 198 °C) ExplainAlcohols are covalent molecules;



Molecules June 1 Browse Articles
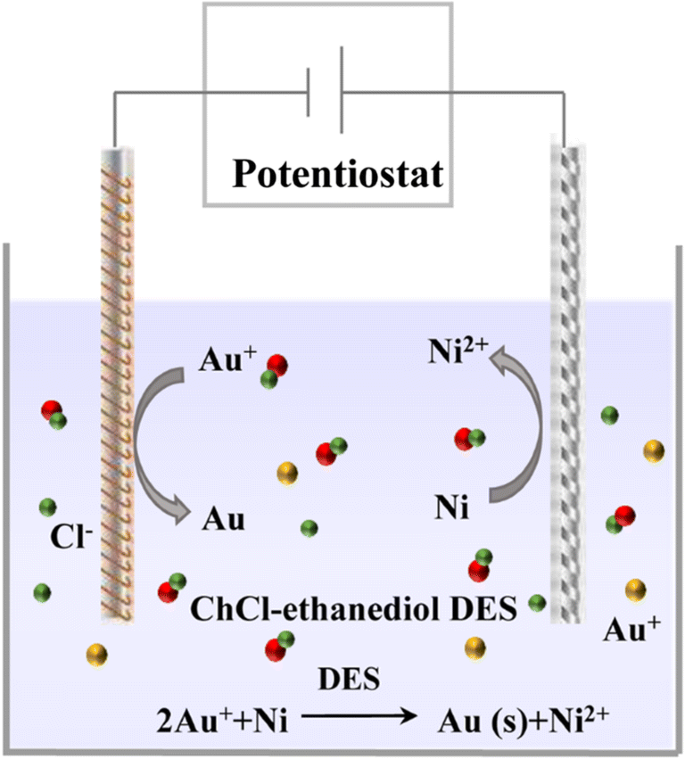



Insights Into The Relationships Between Physicochemical Properties Solvent Performance And Applications Of Deep Eutectic Solvents Springerlink
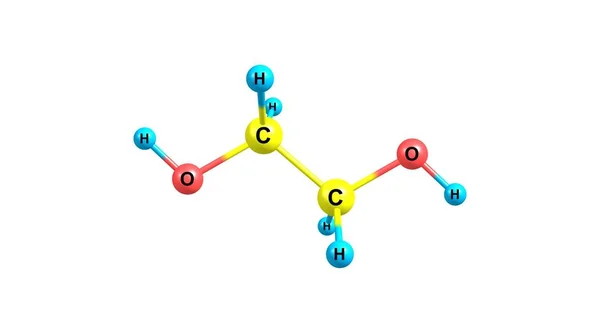



Ethanediol Stock Photos Royalty Free Images Depositphotos



1 2 Ethanediol Structure Shefalitayal
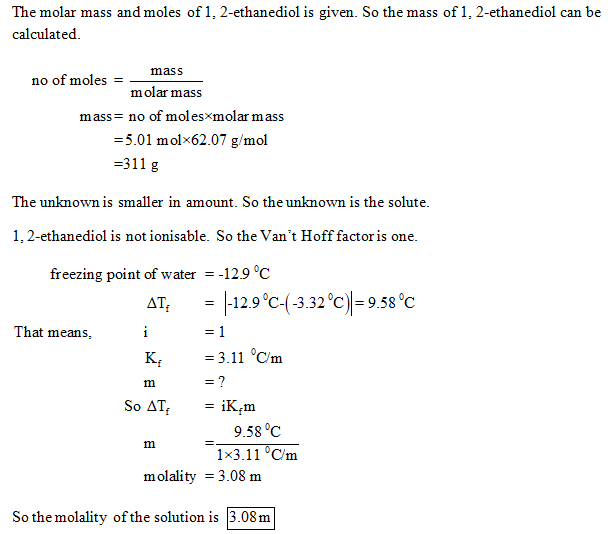



Answered 24 9 G Of An Unknown Molecular Solute Bartleby



Ijms Free Full Text Effectiveness Of Magnolol A Lignan From Magnolia Bark In Diabetes Its Complications And Comorbidities A Review



How Can I Write The Lewis Dot Structure For C2h6 Socratic



1 1 Ethanediol C2h6o2 Chemspider




Solved Change 1 2 Ethanediol Into 1 2 Ethenediol By Removing Chegg Com




1 Introduction To Organic Chemistry 2 Organic Chemistry Difficult Challenging Memorization Chapter Not Well Maybe Body Of Knowledge Application Ppt Download



Frontiers Improving The Post Polymerization Modification Of Bio Based Itaconate Unsaturated Polyesters Catalyzing Aza Michael Additions With Reusable Iodine On Acidic Alumina Chemistry




Pdf On The Extent Of Intramolecular Hydrogen Bonding In Gas Phase And Hydrated 1 2 Ethanediol Semantic Scholar



2




4 Build A Model Of The 1 2 Ethanediol Molecule A Identify The Electron Pair And Molecular Geometry Around Homeworklib



2



Stereoselective Polymerization Of Methyl Methacrylate And Rac Lactide Mediated By Iminomethylpyridine Based Cu Ii Complexes Rsc Advances Rsc Publishing




File 1 1 Ethanediol Png Wikimedia Commons




Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O




1 1 Ethanediol Structure C2h6o2 Over 100 Million Chemical Compounds Mol Instincts
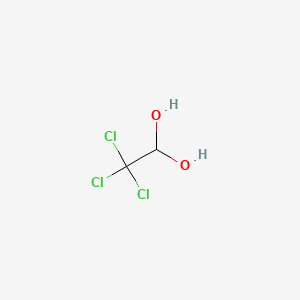



Chloral Hydrate C2h3cl3o2 Pubchem




Solved Chem 234 Spring 18 Pre Lab Worksheet Cyclic Acetal Chegg Com




Molecule Gallery Alcohols




Pushing Curly Arrows
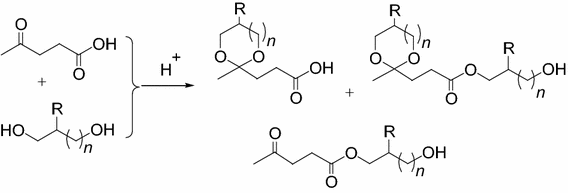



Acid Catalyzed Competitive Esterification And Ketalization Of Levulinic Acid With 1 2 And 1 3 Diols The Effect Of Heterogeneous And Homogeneous Catalysts Springerlink
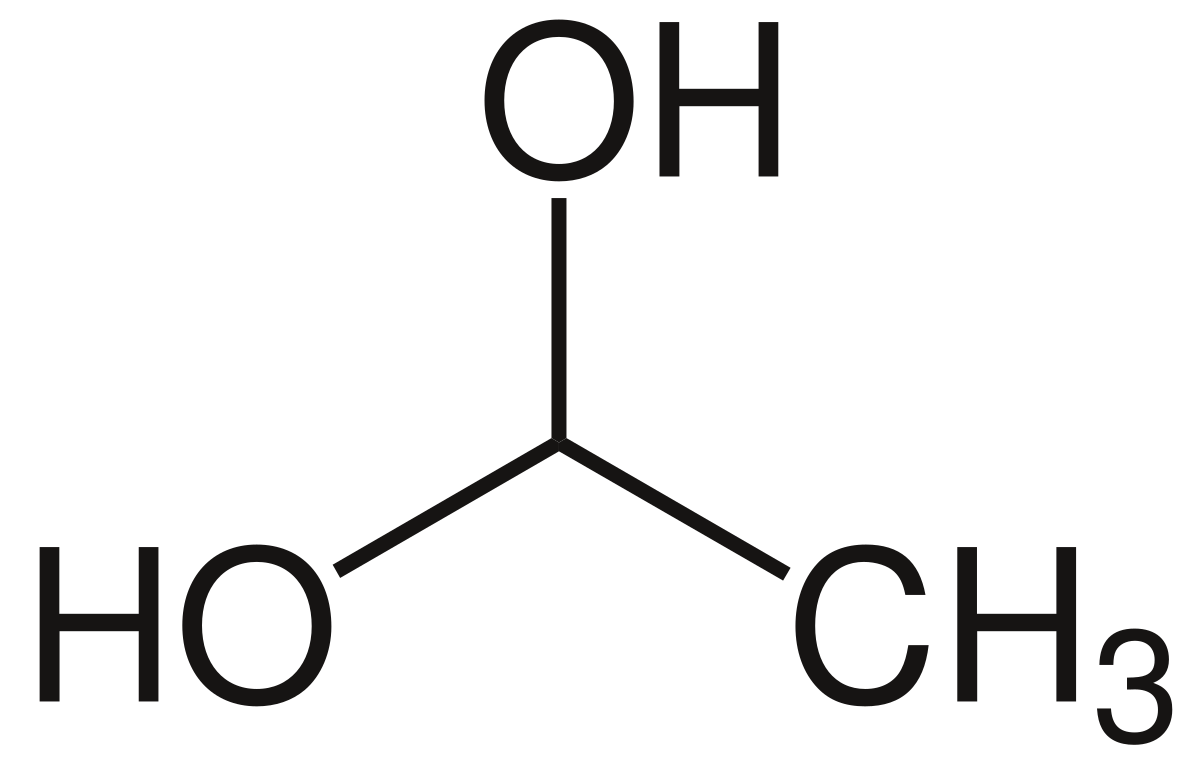



File 1 1 Ethanediol Svg Wikimedia Commons




X Ray Molecular Structure Of Dimim 2 Fe 2 Cl 6 M O 2 Thermal Download Scientific Diagram




Supramolecular Assembly Of L Lysine On Zsm 5 Zeolites With Different Si Al Ratio Sciencedirect




Alcohols And Ethers




Ethylene Glycol Detection C2h6o2 Gas Factsheet Ion Science Uk




Influence Of Interparticle Electronic Coupling On The Temperature And Size Dependent Optical Properties Of Lead Sulfide Quantum Dot Thin Films Journal Of Applied Physics Vol 119 No 9




Layered Inorganic Cationic Frameworks Beyond Layered Double Hydroxides Ldhs Structures And Applications Wang European Journal Of Inorganic Chemistry Wiley Online Library




Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O




Catalytic Conversion Of Pure Glycerol Over An Un Modified H Zsm 5 Zeolite To Bio Based Aromatics Sciencedirect
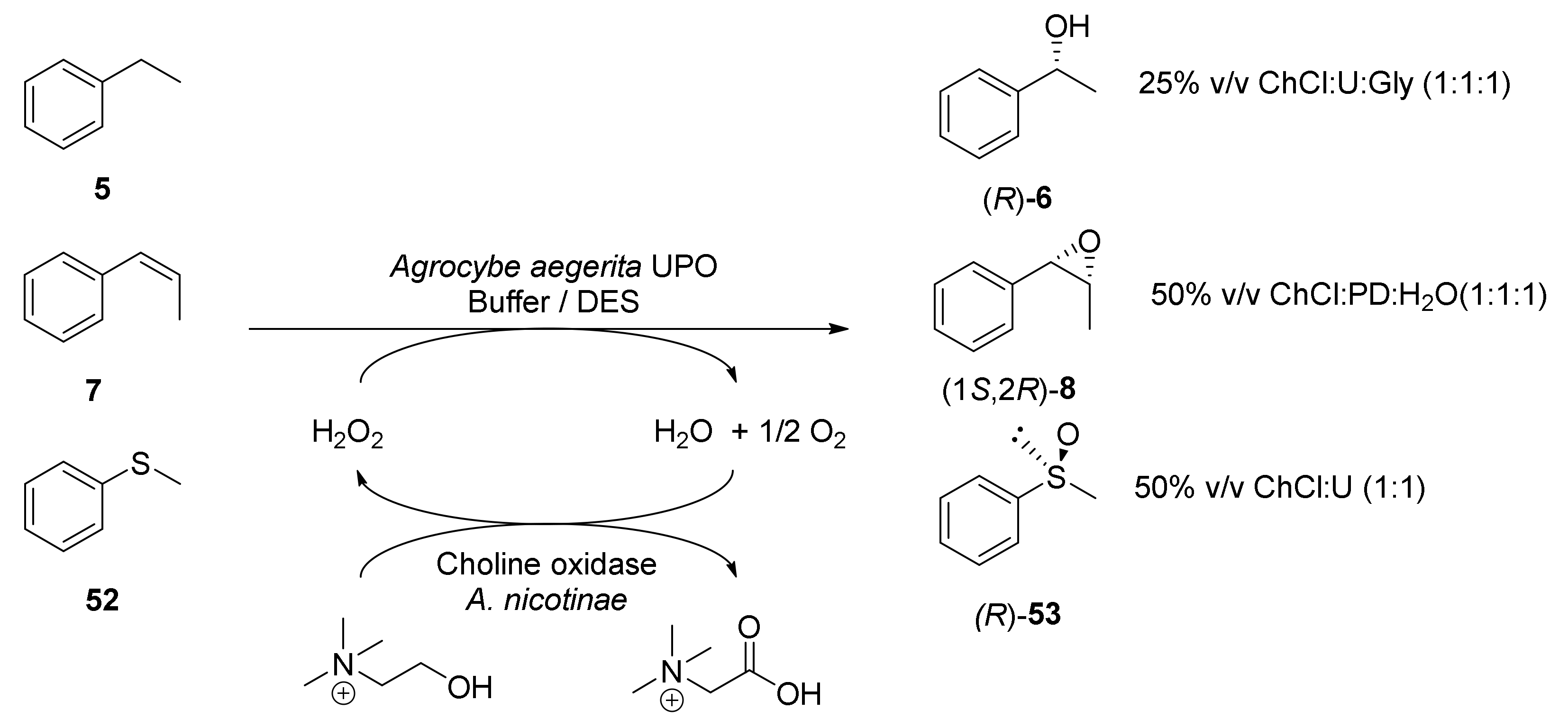



Molecules Free Full Text Biocatalyzed Redox Processes Employing Green Reaction Media Html



Lewis Structure 2 Hexanone Diagram C5h8o2 Pubchem Png 629x433px Watercolor Cartoon Flower Frame Heart Download Free
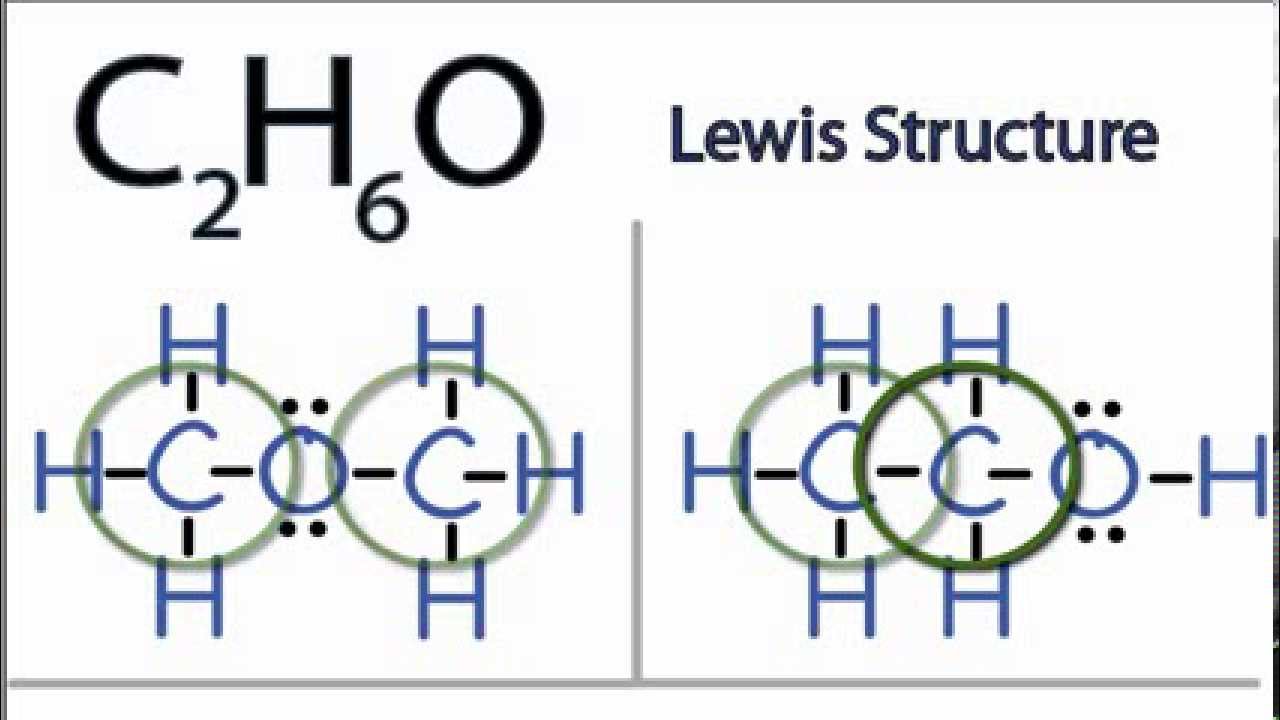



C2h6o Lewis Structure How To Draw The Lewis Structure For C2h6o Youtube




In The Lewis Dot Structure Of Ethylene Glycol Would One Hydrogen Atom Visibly Connect The Two Oxygen Atoms And If So Would One Line Connecting That Same Hydrogen Atom To One Of




Acros Organics Ethylene Glycol 99 1l Cas 107 21 1 1 2 Ethanediol 1 2 Ethanediol From Masterflex
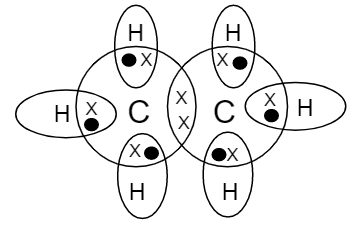



Draw The Electron Dot Structure Of Ethane Class 12 Chemistry Cbse




Pentaerythritol As Efficient H Bonding Organocatalyst For Synthesis Of Indazolo 2 1 B Phthalazine Trione Derivatives Request Pdf




Environment And Climate Change Canada Acts Regulations Ethylene Glycol Final Content



E Organic Chemistry Exercises Chemistry Libretexts
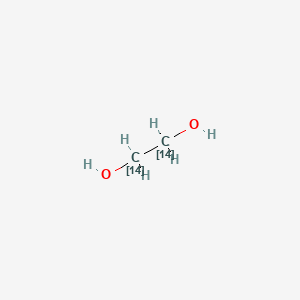



1 2 Ethanediol 1 2 14c2 C2h6o2 Pubchem
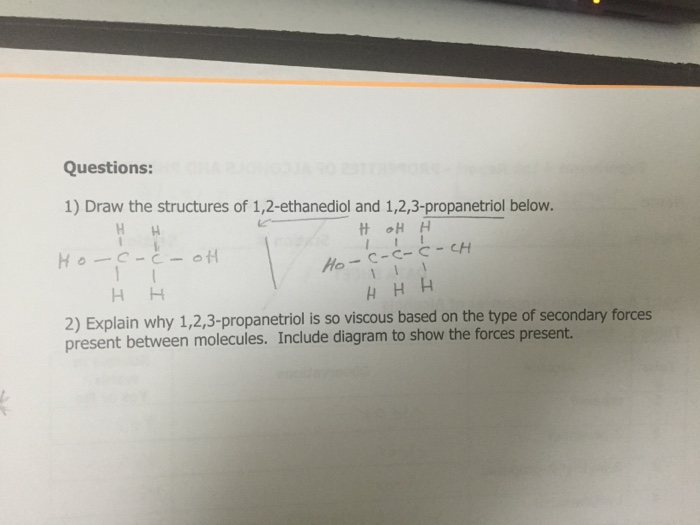



Solved Draw The Structures Of 1 2 Ethanediol And 1 2 Chegg Com
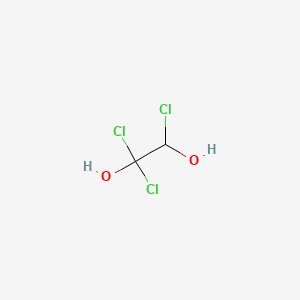



1 1 2 Trichloro 1 2 Ethanediol C2h3cl3o2 Pubchem




1 1 Ethanediol Semantic Scholar



Isonipecotic اسید Cas 498 94 2 تولید کنندگان تامین کنندگان کارخانه صفحه اصلی آفتاب فارما
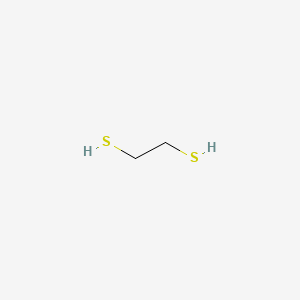



1 2 Ethanedithiol C2h6s2 Pubchem




Conformational Energies For 1 2 Ethanediol And A D Galactose For Which Download Scientific Diagram




Solved Change 1 2 Ethanediol Into 1 2 Ethenediol Hochchoh Chegg Com



1 Propanol Lewis Structure Structural Formula Butanol Png Clipart 2butanol Alcohol Angle Area Black Free Png



1 1 2 Ethanetriol C2h6o3 Chemspider




Alcohols And Ethers Chemistry Atoms First



2



2



1
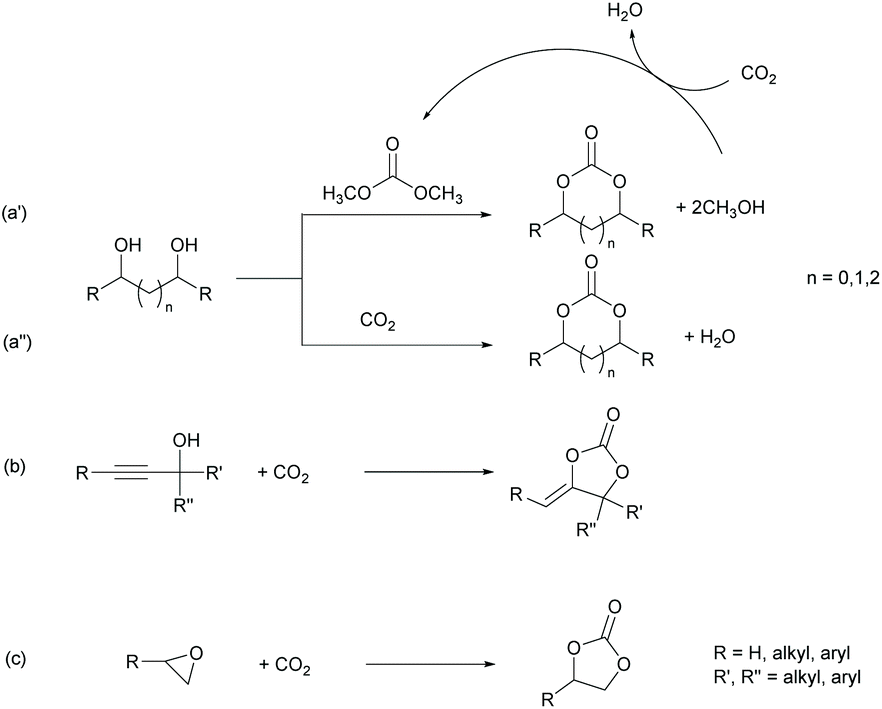



Tandem Catalysis One Pot Synthesis Of Cyclic Organic Carbonates From Olefins And Carbon Dioxide Green Chemistry Rsc Publishing Doi 10 1039 D0gch




B What Are The Bond Angles For H C H For H C C Monteyne Chemistry By Inquiry Course Hero




Organic Chemistry I Homework 1998




Using Deep Eutectic Solvents To Improve The Biocatalytic Reduction Of 2 Hydroxyacetophenone To R 1 Phenyl 1 2 Ethanediol By Kurthia Gibsonii Sc0312 Sciencedirect




Molecule Gallery Alcohols
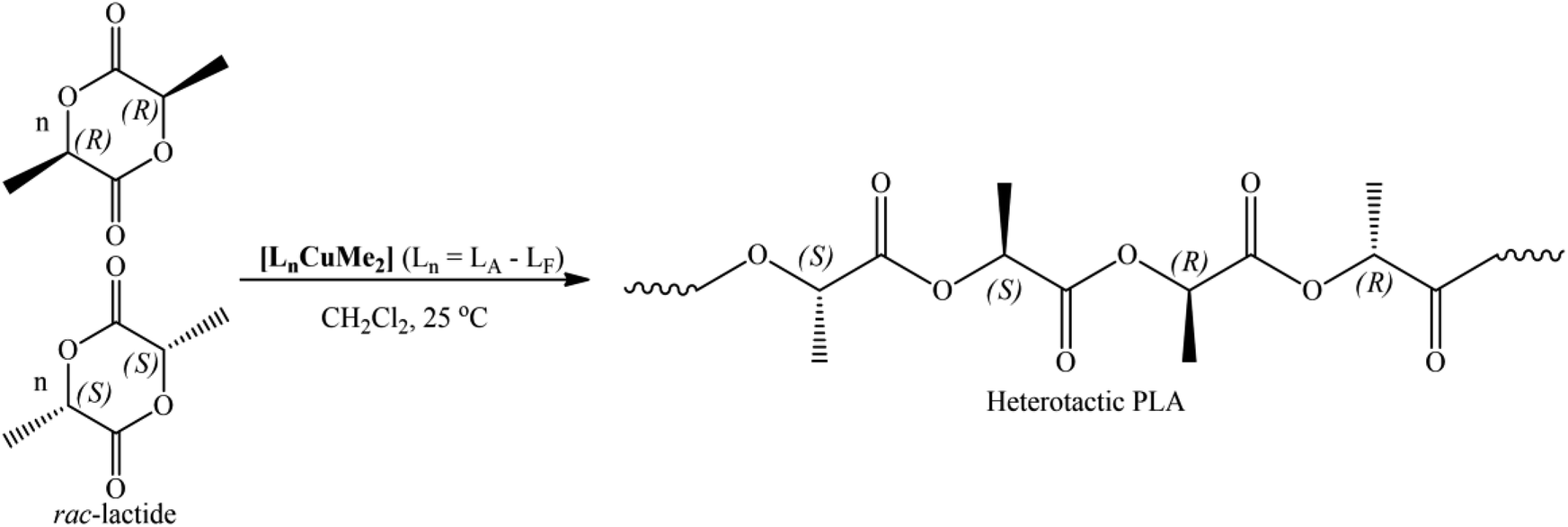



Stereoselective Polymerization Of Methyl Methacrylate And Rac Lactide Mediated By Iminomethylpyridine Based Cu Ii Complexes Rsc Advances Rsc Publishing




A Bifunctional And Recyclable Catalyst Amine And Ionic Liquid Grafting On Mofs For The One Pot Synthesis Of N Aryl Oxazolidin 2 Ones Sciencedirect




Chiral Ionic Liquids Structural Diversity Properties And Applications In Selected Separation Techniques Abstract Europe Pmc
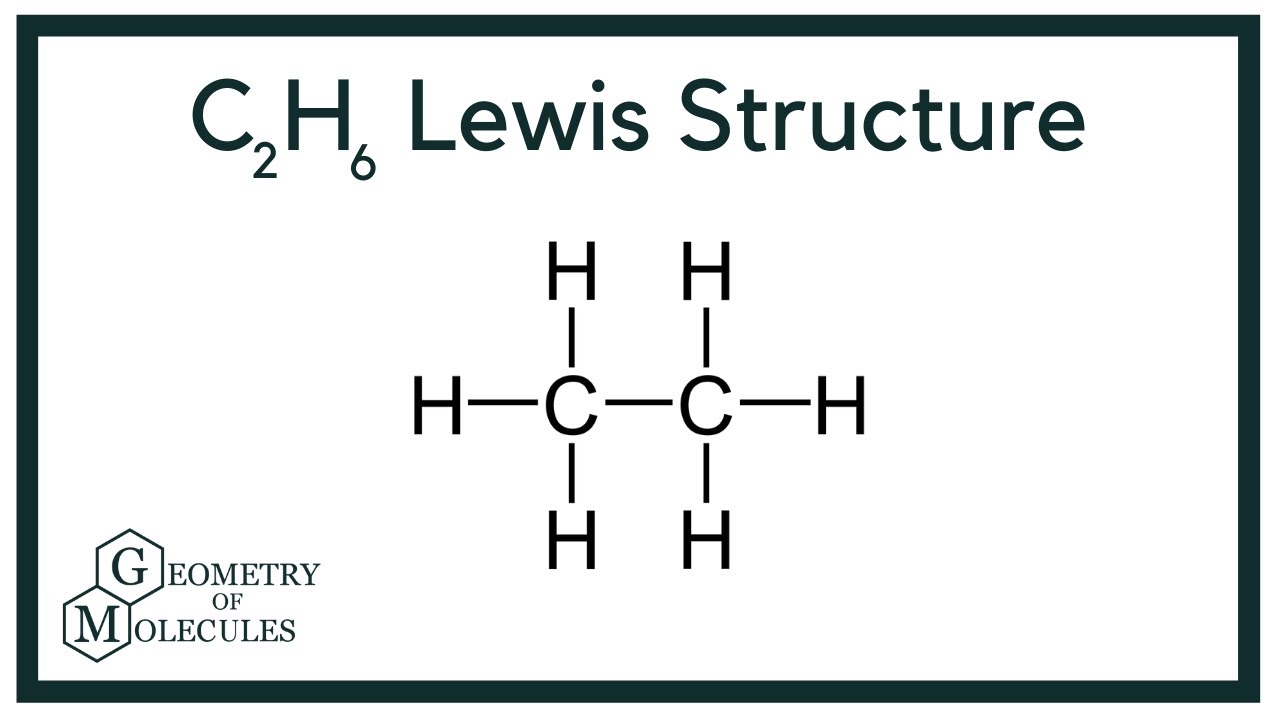



C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape



Pubs Rsc Org




Alcohols And Ethers Chemistry Atoms First



2



Rcsb Pdb 6ncj Structure Of Hiv 1 Integrase With Potent 5 6 7 8 Tetrahydro 1 6 Naphthyridine Derivatives Allosteric Site Inhibitors



Catalytic Application Of Sulfamic Acid Functionalized Magnetic Fe3o4 Nanoparticles Sa Mnps For Protection Of Aromatic Carbonyl Compounds And Alcohols Experimental And Theoretical Studies Rsc Advances Rsc Publishing



Solved Use This Condensed Chemical Structure To Complete The Chegg Com



Rcsb Pdb 7pi4 Fak Protac Gsk215 In Complex With Fak And Pvhl Elonginc Elonginb



Chemical Product Catalog Letter H Page 265 Chemicalbook




X Ray Molecular Structure Of Dimim 2 Fe 2 Cl 6 M O 2 Thermal Download Scientific Diagram




Solved Change 1 2 Ethanediol Into 1 2 Ethenediol By Removing Chegg Com



What Is The Electron Dot Structure Of Ethane Quora



C2h6 Lewis Structure Etane Hybridization Molecular Geometry And Shape




1 1 Ethanediol Semantic Scholar




1 1 Ethanediol Semantic Scholar



1 2 Ethanediol



0 件のコメント:
コメントを投稿